 |
2003 Greenland Tour Geo-Adventure Report:
Experience a virtual tour through
the Ilimaussaq Complex written by one of 2003’s Geo-Adventure
participants. Lots of great scenery pics, rock pics
and a simply excellent story.
Buying a new Portable UV
Field Lamp?
Check out our FAQ to help
you decide what features are important to you.

Article (in HTML) - The Fluorescent
Minerals
of the Ilimaussaq Complex,
South Greenland or click on image for a PDF File

Iceberg Arches
|
|
|
Fluorescence in Minerals |
There are around 4,000 different types of
minerals - approximately 15% of them are known to fluoresce.
Impurities in the mineral (usually) cause this fluorescense -
very few “pure” minerals are known to fluoresce.
These impurities - “activators” - are the reason
for different colors. But the presence of an activator does
not mean the mineral will fluoresce - different minerals with
the same activator may even fluoresce different colors.
Some activators require another “coactivator” to cause
fluorescence, and some impurities will quench (prevent) fluorescence.
The bottom line - a piece of fluorite from one location may fluoresce
brightly while one from another location may not fluoresce at
all.
Known activators include: Manganese, Chromium,
Iron, Titanium, Copper, Lead, Europium, Cerium, Uranyl, Tungstate,
Molybdate, Sulfur, Nitrogen, and various Organic activators.
The few minerals that fluoresce when pure
are called ‘self-activated’ minerals. These include
Scheelite, Powellite, and many Uranium minerals. Two of these
minerals are perhaps the primary reason the Fluorescent Mineral
hobby exists today. Both Scheelite and Uranium were important
minerals during WWII. Prospectors used ultraviolet lights to hunt
out deposits of both minerals.
|
|
A Sampling of Fluorescence caused by Major
Activators |
|
Scheelite, a major ore of tungsten,
usually is recognized by its brilliant sky-blue fluorescence.
Molybdenum as a coactivator modifies the color to white
or yellow. |
 |
|
Several secondary uranium minerals,
such as autunite, characteristically fluoresce a bright
yellowish green. Caused by the uranyl ion, this ion is
so prone to fluorescence that trace amounts of it cause
yellowish-green fluorescence in a very large number of
minerals, including adamite, apophyllite, aragonite, calcite,
quartz, and opal. |
 |
|
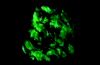
Willemite, a zinc mineral, usually
fluoresces a bright green. This is due to traces of manganese.
|
 |
|
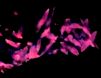
Calcite fluoresces in most all colors
due to different activators. Red and pink fluorescent
calcites can be activated by lead and manganese. Green
fluorescence is due to uranyl ion traces. Calcite from
the mercury mines at Terlingua, Texas fluoresces pink
under longwave UV and bright blue under shortwave UV.
It also has a bright blue phosphorescence after the UV
lamp is removed. |
 |
|
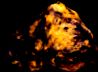
Many Fluorites fluoresce a blue-violet
color due to traces of europium; this is usually best
under longwave UV. Fluorite also fluoresces green, yellow,
red, and white. Some will fluoresce one color under short-wave,
a second color under longwave, and even a third phosporescense.
Other activators in Fluorite include Yttrium, Samarium,
and some organic impurities. |
 |
|
Scapolite (or Wernerite) from Quebec,
Canada, fluoresces a vivid orange-yellow color under longwave
UV, while short-wave UV causes a bright phosphorescence. |
 |
|
Tugtupite from Illimaussaq, Greenland
is a rare but beautiful minerals only found in a few places
in the world. Under Longwave it fluoresces a salmon red
color and a deep cherry-red under Short-wave. |
 |
|
|









